US 10,786,184
Method For Determining Hearing Thresholds In The Absence Of Pure-tone Testing
Patent Number
Issue Date
Inventor(s)
Joseph H. Bochner;
Wayne M. Garrison
Document
Download PDF for patent US 10,786,184Synopsis
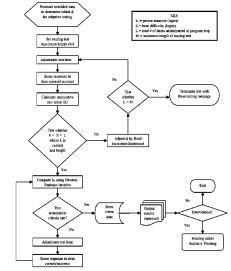
Patent US 10,786,184 B2 describes a method for determining hearing thresholds without requiring traditional pure-tone audiometry. This invention introduces an adaptive speech recognition test coupled with statistical modeling to provide an efficient and accessible way to assess hearing loss.
The novel aspects of this invention stem from its departure from conventional pure-tone testing, which can be time-consuming and require specialized equipment and trained personnel. Instead, the method utilizes an adaptive speech recognition test that incorporates a calibrated pool of test items, each with a known difficulty value and standard error. Responses from individuals undergoing the test are then analyzed using multiple regression statistical methods. This statistical analysis develops an equation that converts an individual's speech recognition test score into hearing threshold values across several frequencies. The same adaptive speech recognition test can subsequently be administered to other individuals, and their scores can be directly translated into hearing thresholds using the established equation.
This approach offers several key innovations:
Absence of Pure-Tone Testing: It eliminates the need for pure-tone audiometry, making hearing assessment more accessible, especially in environments lacking specialized audiology clinics or trained audiologists.
Adaptive Testing: The adaptive nature of the speech recognition test means that the difficulty of presented items adjusts based on the individual's responses, making the testing process more efficient and tailored to the individual's hearing abilities.
Statistical Conversion: The use of multiple regression to directly convert speech recognition scores to hearing thresholds at various frequencies provides a robust and validated method for hearing assessment.
The commercial potential of this invention is significant, particularly in expanding access to hearing healthcare and streamlining diagnostic processes.
Possible applications include:
Primary Care and Telemedicine: This method could enable general practitioners, nurses, or other healthcare providers to conduct preliminary hearing screenings in their offices without the need for an audiologist, facilitating earlier detection of hearing loss. It is particularly well-suited for telemedicine platforms, allowing remote hearing assessments.
Public Health Screening Programs: The simplified nature of the test makes it highly scalable for large-scale hearing screening programs in schools, workplaces, or community centers, especially in underserved or remote areas where access to traditional audiology services is limited.
Direct-to-Consumer Hearing Solutions: Companies developing over-the-counter (OTC) hearing aids or personal sound amplification products (PSAPs) could integrate this technology to provide users with a convenient and accurate way to self-assess their hearing, enabling better customization of devices.
Military and Industrial Health: For occupations with high noise exposure, regular and efficient hearing monitoring is crucial. This method could offer a more practical and frequent screening tool to track changes in hearing thresholds.
Resource-Limited Settings: In developing countries or areas with limited healthcare infrastructure, this invention could revolutionize access to hearing assessment, allowing for widespread screening and identification of individuals needing intervention.
This technology provides a practical, scalable, and validated method for determining hearing thresholds, addressing a critical need for accessible and efficient hearing assessment solutions across various sectors.