Research
- RIT/
- Biomechanical Imaging Lab/
- Research
- Current Research
- Ultrasound Elastography of the Vocal Folds
- General Methods for Elastic Inverse Problems
- Risk Assessment of Abdominal Aortic Aneurysm
- Ultrasound Elastography of Achilles Tendinopathy
- Ultrasound Imaging Biomarkers for Plantar Heel Pain
- Previous Research
- Development of a Longitudinal, Non-invasive Ultrasound Technique to Monitor Tendon Healing in a Mouse Model
Ultrasound Elastography of the Vocal Folds
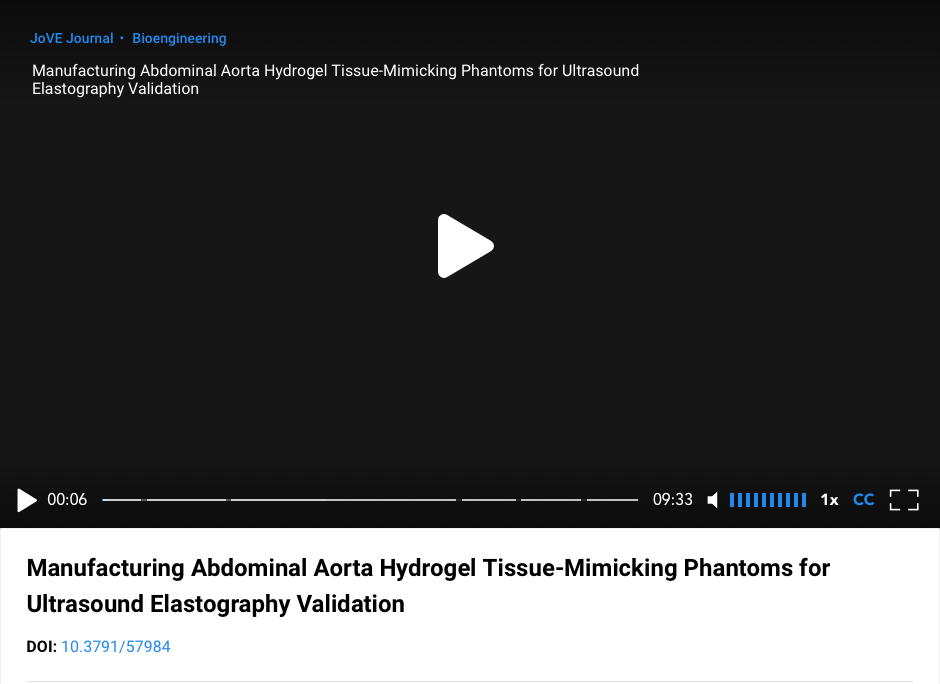
The primary source of voice production in humans is the vibration of the vocal folds (VF) of the larynx, which is characterized by the fundamental frequency of phonation (FFP). Precise control of the FFP is obtained by contraction of laryngeal muscles, which changes the tension on the VF’s, called VF posturing, and modification of subglottal respiratory pressure. The overall prevalence of voice disorders is relatively high, particularly among professions that rely on proper voice production. While ex vivo experiments and computational models can help elucidate the role that muscle and respiratory control play in modifying the FFP, patient-specific clinical voice assessment is still required to diagnose dysfunction, plan treatment strategies, and monitor patient recovery following surgeries or other therapies. However, current noninvasive methods for evaluating these properties in living patients are quite limited. This project proposes to develop a novel US elastography technique to allow for the non-invasive, transcutaneous measurement of patient-specific, non-linear elastic VF mechanical properties with increasing FFP on a live subject who can provide biofeedback during evaluation. The study incorporates both forward and inverse finite element modeling, shear wave elastography, and techniques for tracking dynamic motion. These components work together to reconstruct material properties based on tissue movement. We hypothesize that the measurement of VF vibrations will allow us to study the non-linear relationship between the apparent VF tissue dynamic stiffness and the FFP, and that characterizing this relationship will lead to a paradigm shift in the diagnosis of a range of voice disorders, which could lead to more effective treatment and better outcomes for patients with voice disorders. Once validated, the clinical utility of our technique will be established and will pave the way for future studies on at-risk patient populations or patient populations with specific, identified pathologies.
General Methods for Elastic Inverse Problems
General Methods for Elastic Inverse Problems
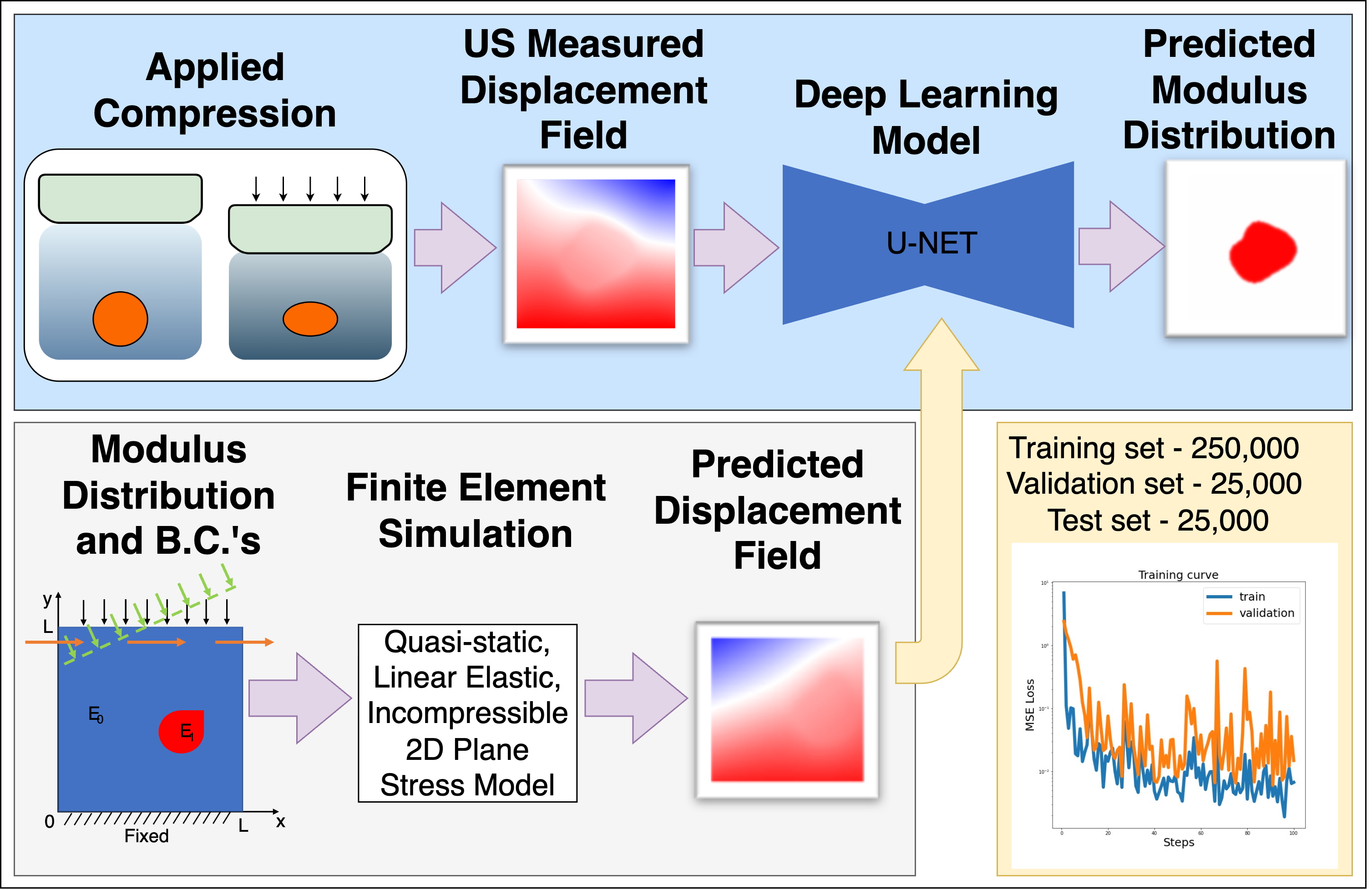
Biomechanical Imaging (BMI) is a technique that expands upon the medical imaging practice of elastography to solve the inverse problem in solid mechanics. This technique takes displacement measurements as input and produces an estimate of the mechanical properties as an output, which can provide a non-invasive means of characterizing tissue structure and function. Potential areas of application span both clinical (e.g., detection and diagnosis of cancerous tumors, atherosclerotic plaques, aneurysms, musculoskeletal disorders, etc.) and laboratory settings (e.g., research tools for physiology and mechanobiology). Displacement measurements are commonly obtained by registration of sequential images of deforming tissue, acquired using medical imaging modalities such as ultrasound (US) or magnetic resonance imaging (MRI). In our lab, we typically use two-dimensional (2D) US as it is relatively inexpensive and safe. However, the inverse problem presents numerous challenges that our lab is working to overcome. For example, the elastic inverse problem is ill-posed, and the accuracy of the reconstruction can depend on the amount of input data, boundary condition assumptions, and the choice of regularization. In general, our lab is investigating ways to improve the accuracy and utility of BMI by improving the models, methods, and assumptions of BMI with a particular focus on clinical efficacy.Recent work in our lab has introduced a deep learning framework for solving the inverse problem in ultrasound elastography. Instead of directly solving governing equations, we train neural networks to learn the mapping between displacement fields and modulus distributions. Using large and diverse datasets generated from forward finite element (FE) simulations, the network trained on these data can generalize to realistic conditions without requiring extensive clinical training data. Specifically, we developed and validated a U-Net-based model trained on simulated axial displacement fields. This model demonstrated accurate recovery of modulus distributions with low error in both simulations and phantom experiments and showed promising preliminary results when applied to clinical data. Our future work in this area will be to incorporate the physical laws of motion into the model training to improve estimates further and ensure they are physically consistent with the measurements.
Risk Assessment of Abdominal Aortic Aneurysm
Risk Assessment of Abdominal Aortic Aneurysm
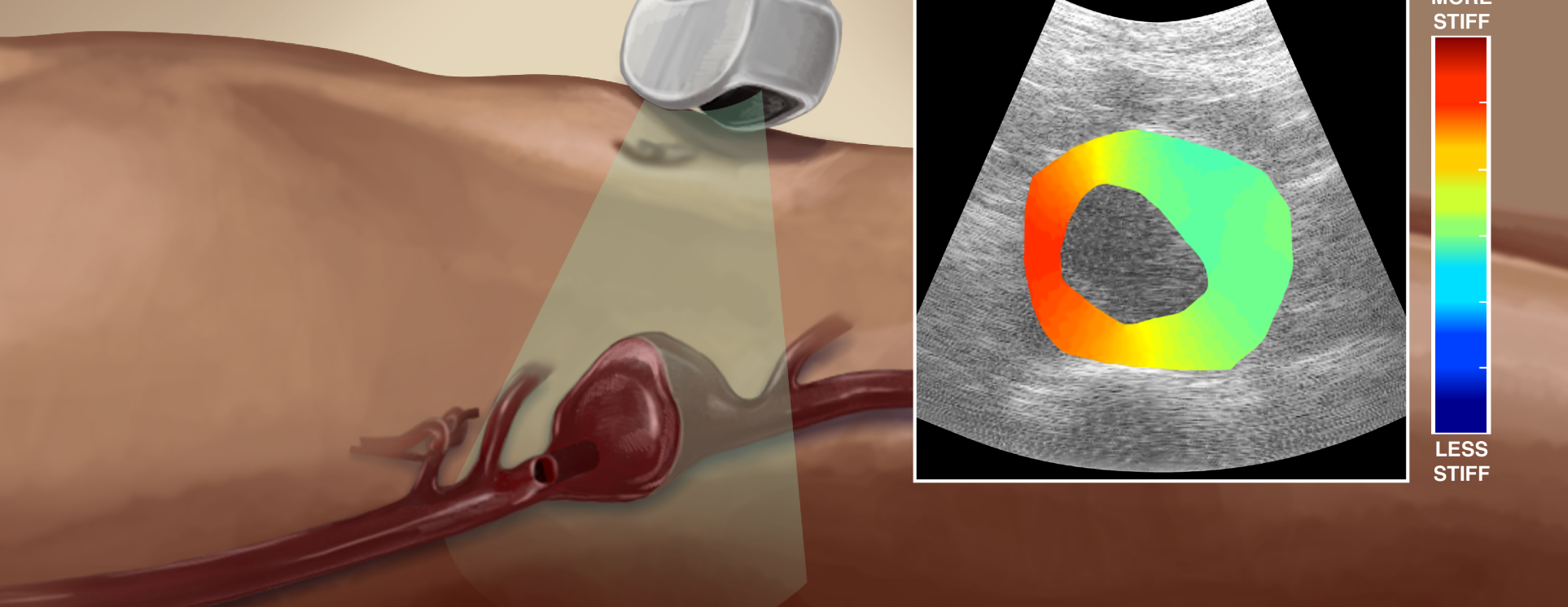
Aneurysms are an excessive localized enlargement of an artery caused by a weakening of the artery wall. For abdominal aortic aneurysms (AAA), clinicians rely on trans-abdominal ultrasound (US) measurements of the AAA maximum diameter. However, the AAA diameter is only a rough estimate of rupture potential. It has been suggested that measurement of stresses within the vessel wall may be crucial factor contributing to aneurysmal stability. Biomechanical imaging techniques can help us measure estimates of the material property changes within aortic tissue and potentially stresses within the wall. This technique is able to measure the aortic tissue stiffness in cross-sectional views of the vessel at the point of maximal diameter. Our lab uses a linear elastic, finite-element model to solve the elastic inverse problem and estimate the shear modulus in that cross-section. This technique also requires a non-invasive blood pressure measurement to estimate the pulse pressure in the aorta and normalize the modulus values. A model based, pulse wave velocity (PWV) technique is also being used to approximate the overall modulus value of the vessel wall in AAA. This technique uses ultrafast US imaging of the aorta in a longitudinal view to approximate the vessel properties.
In the video below (and linked here) we describe a method to manufacture aneurysmal, aortic tissue-mimicking phantoms for the use in testing ultrasound elastography. The combined use of computer-aided design (CAD) and 3-dimensional (3D) printing techniques produce aortic phantoms with predictable, complex geometries to validate the elastographic imaging algorithms with controlled experiments.
Ultrasound Elastography of Achilles Tendinopathy
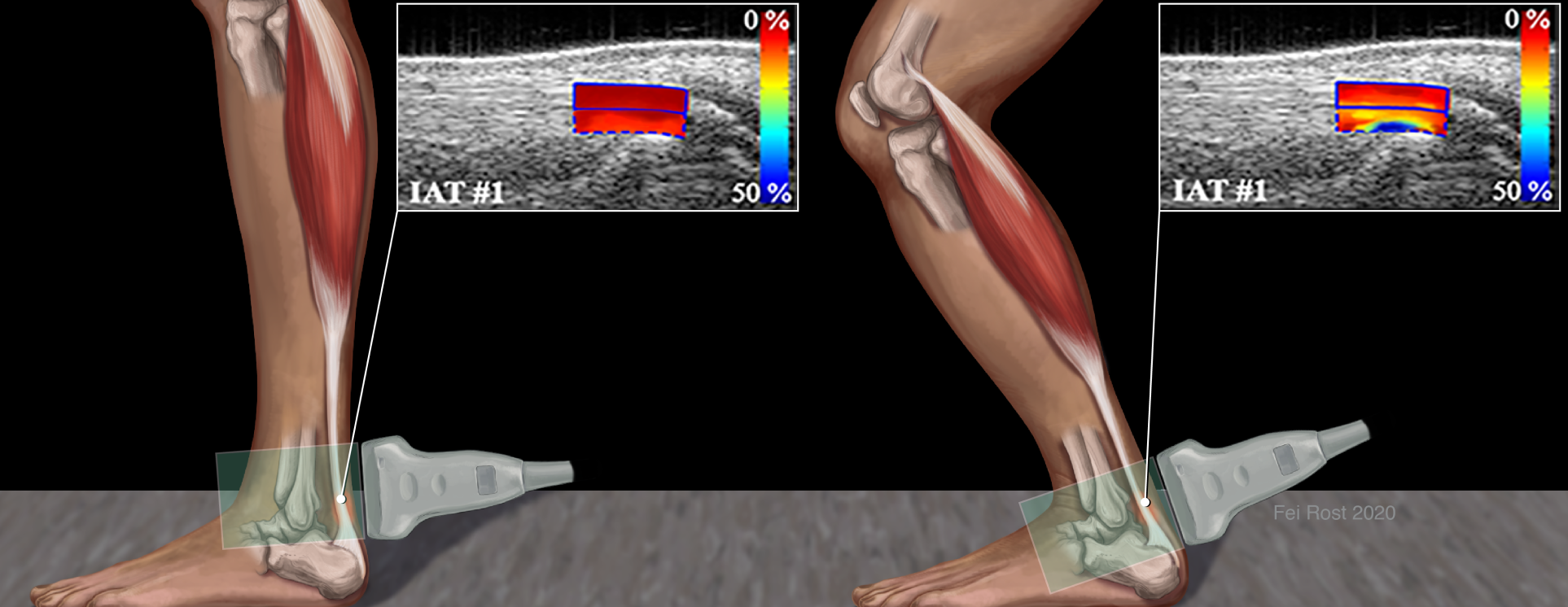
Ultrasound Elastography of Achilles Tendinopathy
Heel lifts are commonly prescribed to patients with Achilles tendinopathy with the general thought that it would relieve tendon stresses during walking to allow the tendon to heal. Yet little is known about the impact of tendon stress on the risk of injury or the healing process. The objective of this project is to demonstrate a connection between various biomechanical characteristics of tendons assessed in vivo via US imaging, including slack, buckling strain and stress in the Achilles tendon, to risk factors for tendinopathy. This work aims to develop an ultrasound elastography measurement protocol and algorithm to measure compressive strain of human Achilles tendon in vivo during certain exercises as well as the non-linear mechanical properties of the tendons for at risk populations. For example, it is well known that the risk of tendinopathy increases with age. It is our hypothesis that by understanding the mechanical properties and strains in the tendon as a function of aging, we may better understand the cause of this increased risk and potentially develop novel therapies based on our findings. Our techniques utilize common elastographic techniques such as quasi-static, quantitative elastography, measurement of large strains and shear wave imaging of tendons.
Ultrasound Imaging Biomarkers for Plantar Heel Pain
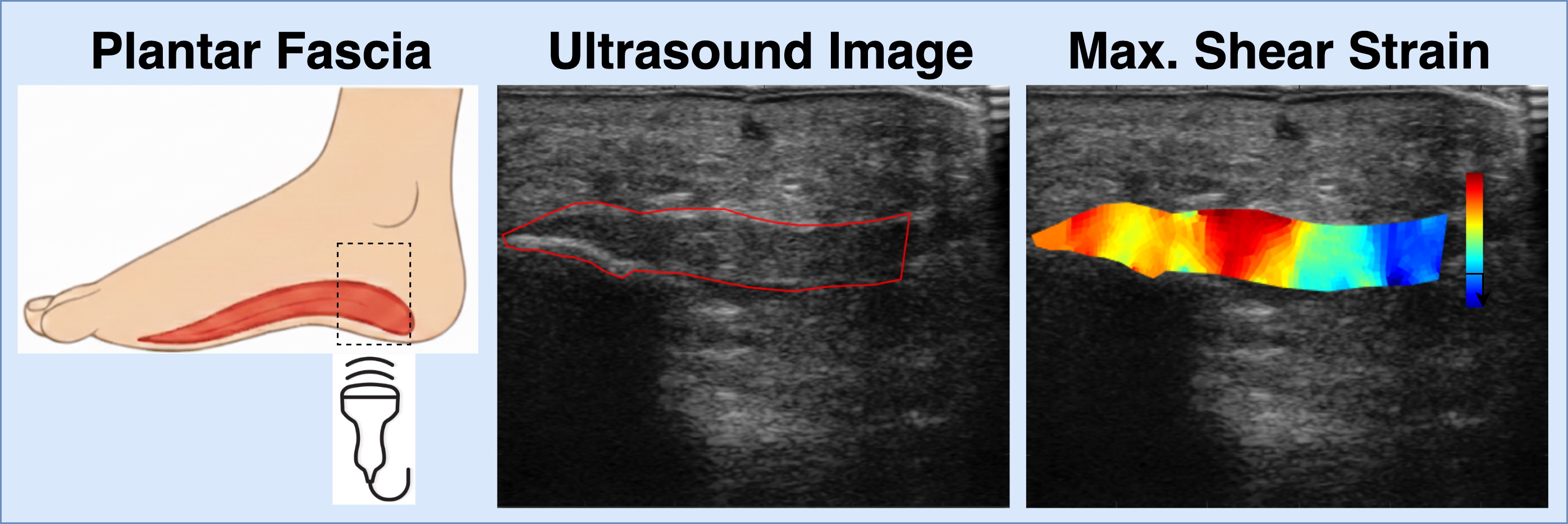
Development of Diagnostic and Prognostic Ultrasound Imaging Biomarkers for Plantar Heel Pain
Myofascial pain is a common but often underdiagnosed source of musculoskeletal pain. One of the challenges in treating it effectively is the lack of objective tools, or biomarkers, that can detect abnormal tissue and guide personalized treatment. As part of a larger study run out of the University of Iowa, our lab is focused on developing ultrasound-based BMI methods for imaging biomarkers of myofascial pain, with plantar fasciitis as a model. We hypothesize that quantifying the shear strain surrounding the plantar fascia and comparing these values across patients with plantar fasciitis, Achilles tendinopathy, and pain-free controls, we can detect a potential image-based biomarker of abnormal myofascial tissue. Using 2D ultrasound and image registration techniques, we can measure the shear strain within the plantar fascia during controlled exercise. Our goal is to provide a simple, non-invasive way to detect abnormal myofascial tissue and track changes to the tissue with treatment. Ultimately, our goal is to improve musculoskeletal pain management by giving clinicians better imaging tools to diagnose pain mechanisms and tailor therapies for each patient.
Development of a Longitudinal, Non-invasive Ultrasound Technique to Monitor Tendon Healing in a Mouse Model
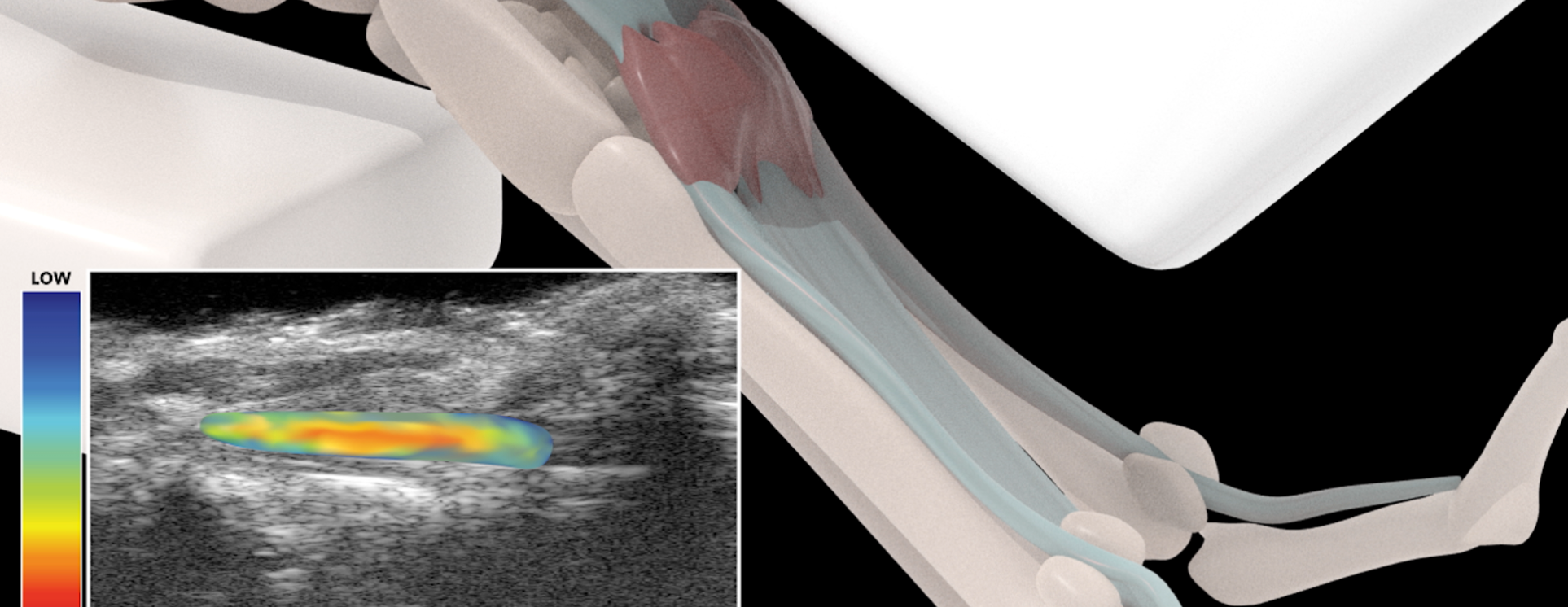
Development of a Longitudinal, Non-invasive Ultrasound Technique to Monitor Tendon Healing in a Mouse Model
Following surgical repair, tendon injuries heal with function-limiting and mechanically insufficient scar tissue. Despite attempts using a variety of biological and tissue engineering approaches, there is currently no consensus therapy to improve outcomes after tendon injury. An insufficient understanding of the underlying mechanisms that contribute to scar-mediated tendon healing is a major impediment to the development of successful therapies. To address this limitation, our collaborators use a murine model of tendon injury and repair that recapitulates many clinical aspects of healing including abundant scar tissue formation and impaired restoration of mechanical properties. However, there remains a need for cost-effective longitudinal outcome measures of tendon healing. For example, restoration of tendon gliding function, mechanical properties and tissue morphology are assessed by end-point analyses, which requires sacrificing the mice, and destructive testing of the tissue. Thus, current approaches are expensive, time-consuming and do not allow longitudinal evaluation or concomitant assessment of function and tissue morphology in the same specimen. This project aims to develop longitudinal, non-invasive metrics of tendon healing by combining ultrasound (US) elasticity imaging and novel image registration methods. US is an ideal modality to longitudinally assess tendon healing, as it is non-ionizing, and can be easily scaled between pre-clinical and clinical applications. Specifically, we are developing quantitative US outcomes of scar tissue volume, tendon excursion and tendon strain. Our hypothesis is that these metrics strongly correlate with terminal end-point parameters, and can therefore serve as faithful biomarkers of tendon healing. Once validated, this technology will permit the rapid screening of biological and pharmacological interventions for healing, and identify promising therapeutic targets in an efficient, cost-effective manner.