Research
Current Research
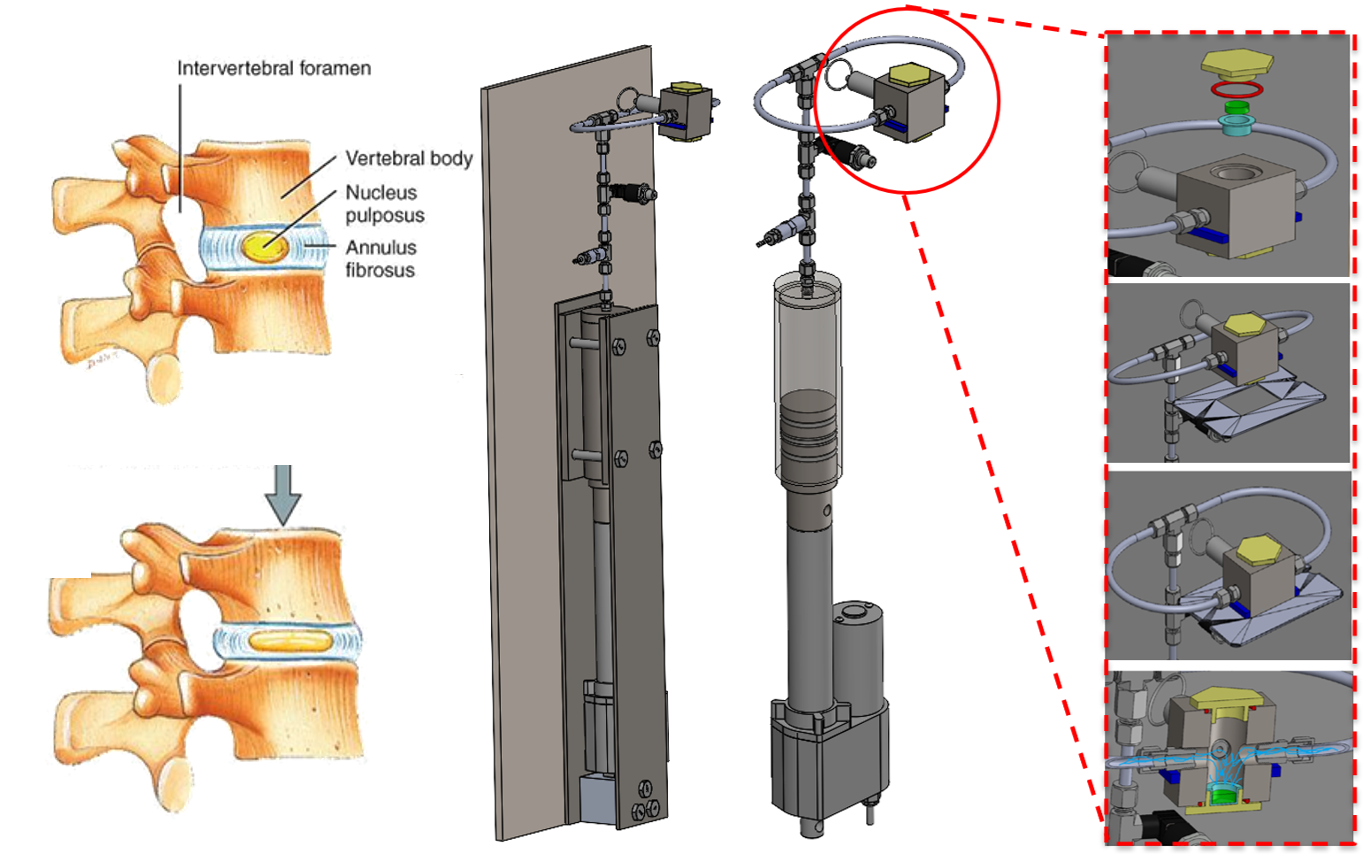
TRP Channel Activity Under Cyclic Compression
Traditional 2D cell culture systems lack key physiological cues, such as the mechanical forces cells experience in the human body. These forces affect development, differentiation, proliferation, and mechanosensitive channel activity (e.g., TRP). Without them, in vitro results often diverge from in vivo outcomes, prolonging clinical trials. To address this, we developed a microscope-mountable compression bioreactor that simulates forces on the nucleus pulposus during spinal compression. The device uses a syringe compression system to apply cyclic loads while enabling live fluorescent imaging of TRP channel activity through calcium signaling. This also supports study of NP mechanotransduction pathways, linking mechanical stimuli to biochemical responses that shape cell behavior.
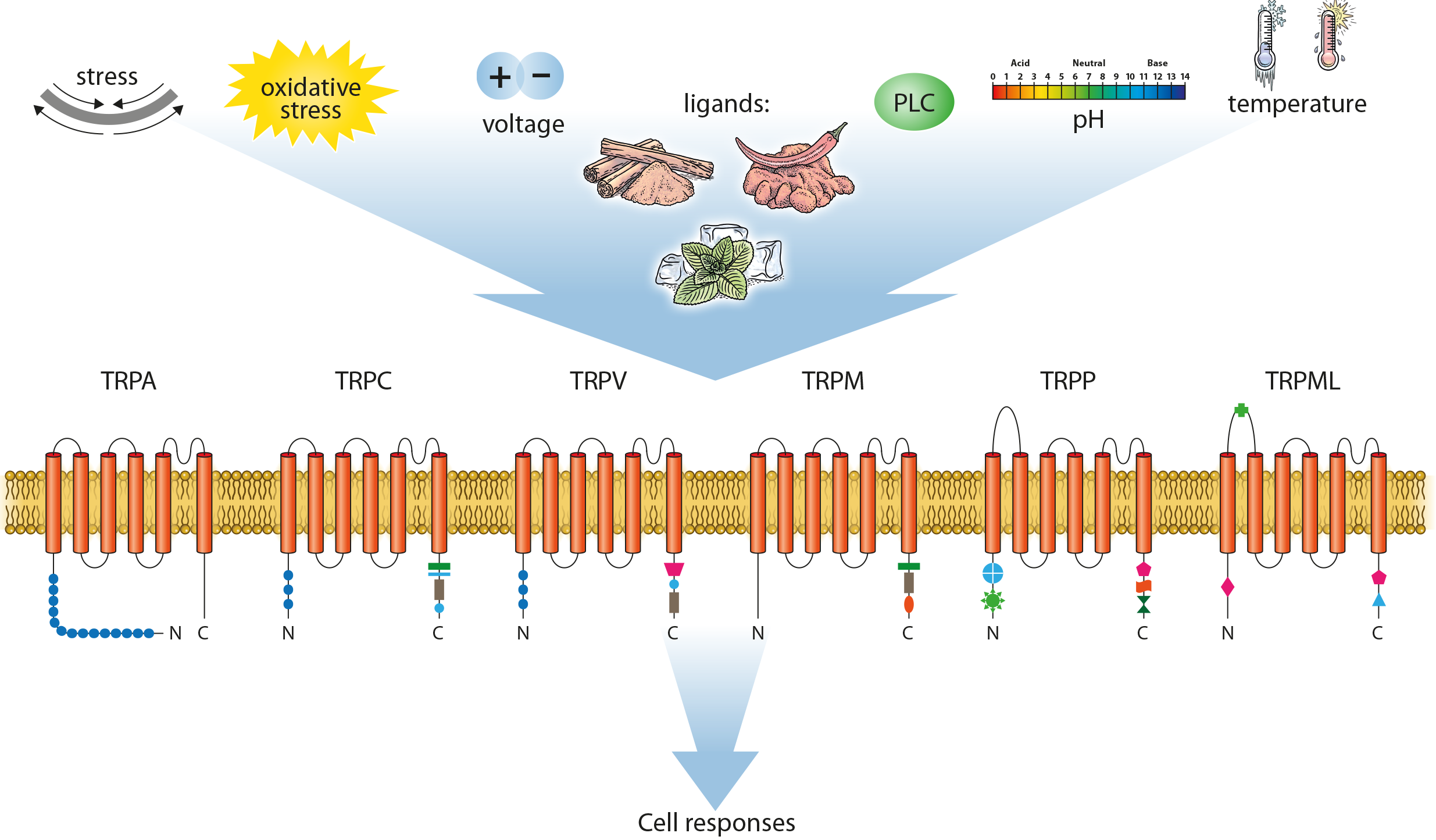
Targeting TRCP6: A Novel Approach for Discogenic Chronic Back Pain
Discogenic chronic back pain (DCBP) is a leading cause of disability, often linked to intervertebral disc (IVD) degeneration, inflammation, and nerve and blood vessel growth. Current treatments, including opioids, are insufficient, highlighting the need for new approaches. This project focuses on TRPC6, a promising target found to be elevated in degenerated, painful IVDs. Our research investigates how TRPC6 is activated within the IVD, its effects on cell behavior, and the potential for using TRPC6 inhibitors as a treatment. By understanding how mechanical and chemical changes in the disc environment influence TRPC6 and testing inhibitors in cell and animal models, we aim to develop a non-opioid treatment for DCBP, providing effective pain relief without addiction risks. If successful, this could lead to significant advancements in treating back pain and improving patient outcomes. Currently funded by Eurospine.
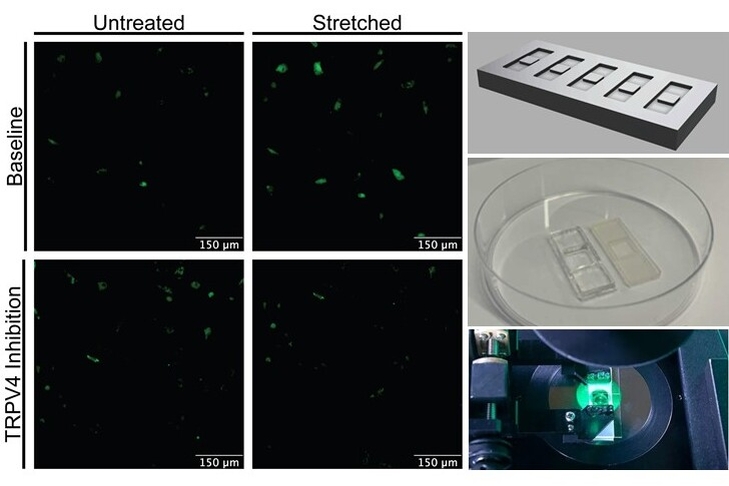
Substrate Stiffness, Topography, and TRPV4 in Disc Mechanotransduction
Over the past decade(s), research has revealed that substrate stiffness and architecture/topography can be recognized by cells, serving as mechanical and topographical cues that ultimately drive cell behavior through mechanoreceptors. These substrate changes can also affect the mechanical stimulation of cells, influencing their response to loading. These responses are largely governed by mechanosensitive ion channels, such as transient receptor potential (TRP) channels. This project aims to determine the impact of substrate stiffness and topography on TRPV4 activation in intervertebral disc cells (AF cells) in response to a pharmacological TRPV4 agonist and cyclic stretching, and its relevance in regulating extracellular matrix synthesis and remodeling. Ultimately, we aim to uncover the role of cell-substrate interactions in intervertebral disc health and disease, leveraging this knowledge to develop regenerative approaches. Currently funded by NIH NIGMS R16 GM146717-01.
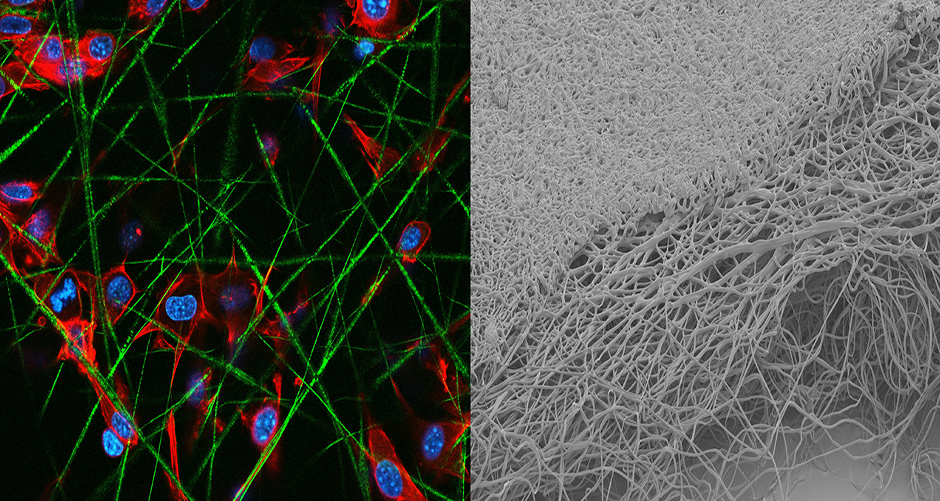
TRPC6 Inhibition for Scleroderma Treatment
Scleroderma is a rare disease characterized by chronic tissue hardening (fibrosis), particularly affecting the skin due to excessive collagen accumulation. Despite extensive research, effective therapies for scleroderma-related skin disease remain elusive. The ion channel TRPC6 plays a significant role in various fibrotic conditions but has not been explored in scleroderma. This project aims to investigate the therapeutic potential of TRPC6 inhibition in treating scleroderma. Initial experiments will utilize classical 2D cell cultures. Concurrently, an electrospun 3D scleroderma skin model will be developed. Cellular cues will induce scleroderma-like conditions in this organotypic model before assessing TRPC6 inhibition effects. Subsequently, the efficacy of TRPC6 inhibition will be evaluated in a bleomycin-induced in vivo scleroderma model. By exploring this novel therapeutic strategy, the project aims to pioneer effective treatments for scleroderma, offering hope for patients grappling with skin hardening and tightening. Currently funded by DoD CDMRP SL20014.
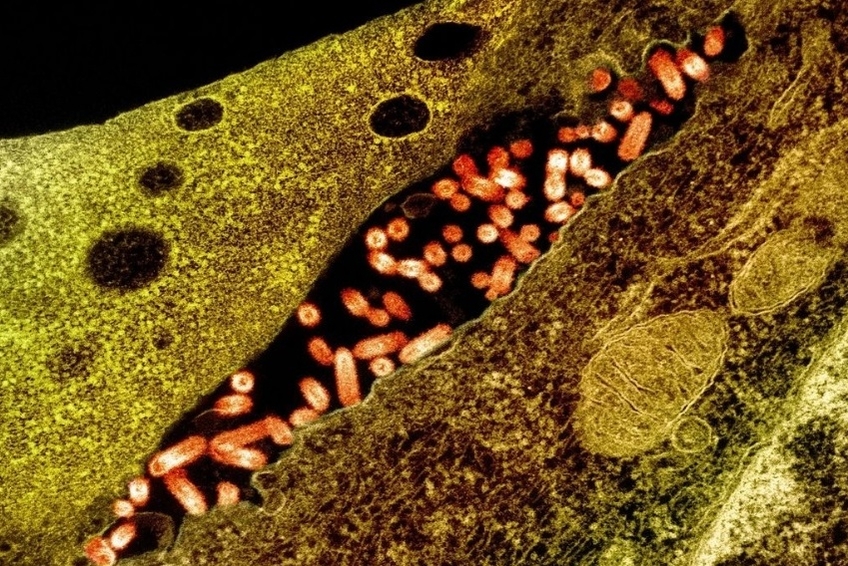
Mechanobiology of Viral Infections
In collaboration with Prof. Dr. Ferran (COS, RIT), we investigate the role of mechanosensitive calcium channels in viral infections, specifically focusing on lung fibroblasts and Vesicular Stomatitis Virus (VSV). By examining how matrix stiffness and mechanical loading influence viral infection dynamics, we aim to uncover the mechanisms by which viruses exploit cellular mechanotransduction pathways. In the future, this research will be further expanded towards investigating the relevance of Toll-like receptors at the intersection of viral infections and cell mechanobiology. This research not only enhances our understanding of viral pathogenesis but also explores potential therapeutic interventions targeting these channels, potentially paving the way for innovative treatments for viral lung infections. Currently funded by NSF 2436976.
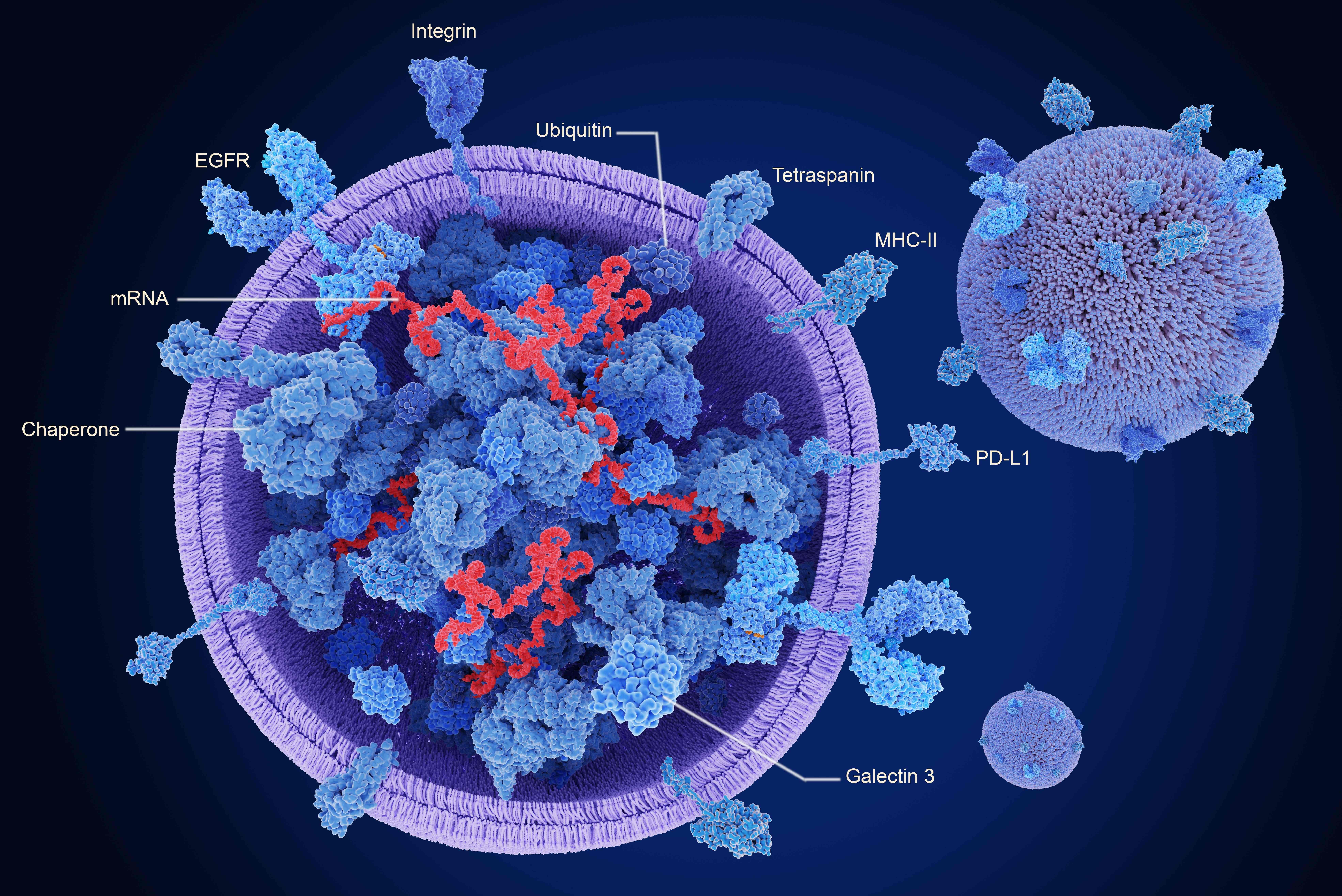
Extracellular vesicles produced by CRISPR-activated MSCs
Degenerative disc disease (DDD) is a leading cause of chronic low back and neck pain, significantly impacting daily activities and work attendance, and creating substantial financial burdens. Traditional treatments, including mesenchymal stem cell (MSC) therapy, face limitations due to the harsh microenvironment of degenerated intervertebral discs (IVDs). This project explores the potential of extracellular vesicles (EVs) derived from MSCs, specifically those enhanced by CRISPR/Cas9 activation of TSG6. By modifying MSCs to overexpress TSG6, we aim to produce EVs with increased therapeutic capacity, targeting inflammation and catabolism in IVD cells. This research aims to develop non-opioid, targeted therapies for DDD, potentially offering new hope for effective pain management and improved quality of life for patients. Currently funded by NIH NIAMS R21AR081987.
Advancing Biomanufacturing of Therapeutic EVs
Extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs) hold immense therapeutic promise, offering benefits such as inflammation inhibition, immune modulation, and tissue regeneration without the risks associated with cell transplantation. However, the practical application of MSC EV therapy is hindered by challenges in scaling up EV production to meet clinical demand. This NSF Future Manufacturing Grant aims to address these challenges by integrating human induced pluripotent stem cells (hiPSCs) and advanced biomanufacturing technologies. The project focuses on leveraging hiPSC-derived MSCs (iMSCs) for scalable EV production, enhancing EV biogenesis through CRISPR activation, and implementing novel nano-membrane technology for efficient EV purification. This collaborative project, conducted with Prof. Dr. Ma (University of Syracuse), Prof. Dr. Gaborski (BME, RIT), and Sartorius, aims establish a robust platform for producing standardized, clinically relevant therapeutic EVs, laying the groundwork for future advancements in regenerative medicine. Currently funded by NSF FMSG 2229111.

Aloe Vera Derived Extracellular Vesicles for Fibrotic Scar Treatment
Hypertrophic scars and keloids, characterized by increased fibrosis and inflammation, are not only aesthetically unpleasing but can also impair daily life activities. Aloe vera gel is known for its diverse medicinal properties. It has been used as an ancient remedy for skin restoration and is increasingly found in pharmaceutical and cosmetic products. However, the therapeutic potential of extracellular vesicles derived from Aloe vera (Av-EVs) remains largely unexplored. In this project, we investigate the anti-fibrotic and immune-modulatory capacities of Av-EVs to determine their relevance for treating fibrotic scars. If successful, we plan to incorporate Av-EVs into a dermal microneedle patch for efficient delivery into the dermis. Ultimately, this research aims to develop a patient-friendly, minimally invasive treatment for fibrotic scars, potentially transforming dermatology practice and significantly improving patients' quality of life.
Research Archive
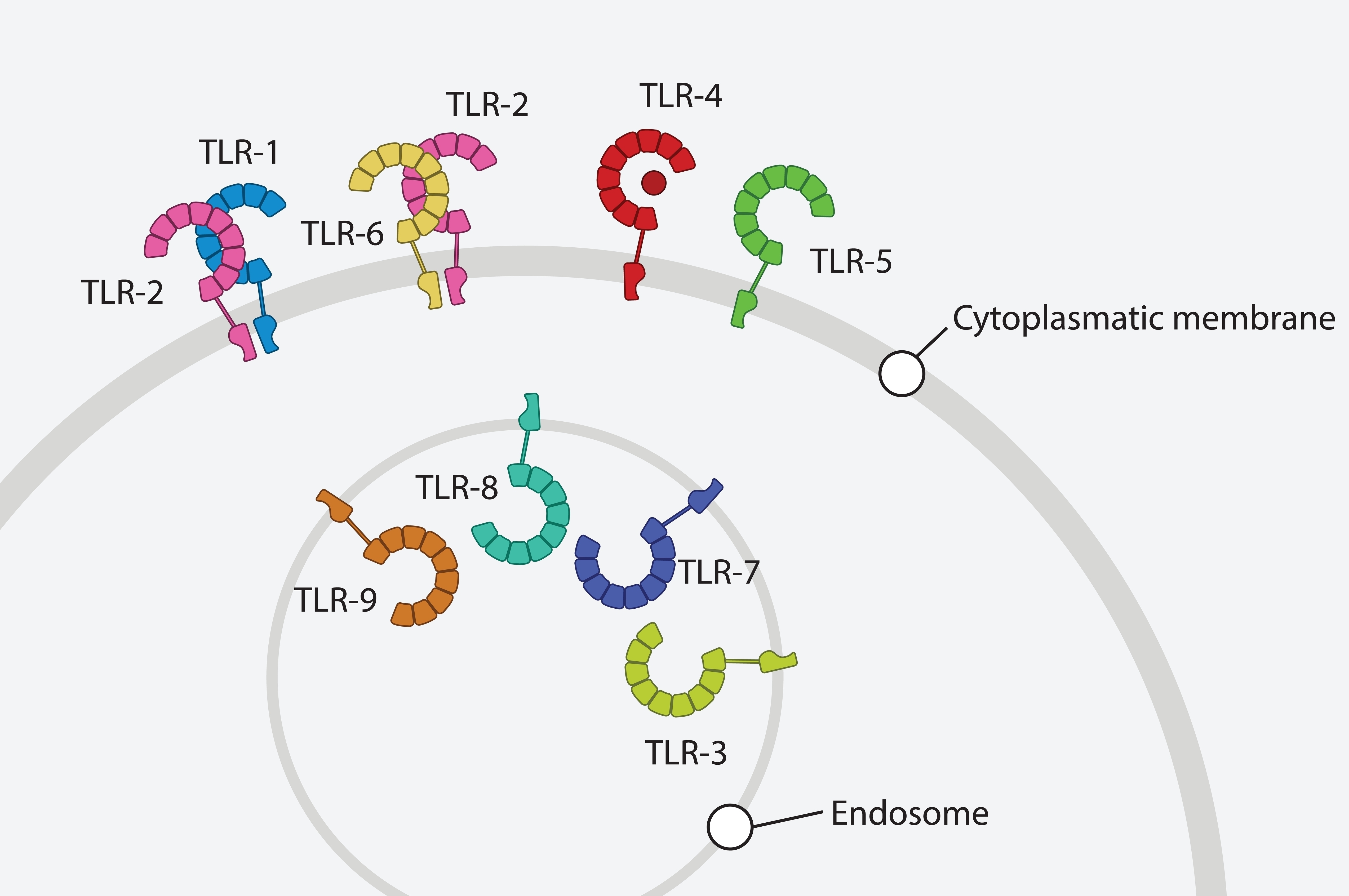
TLR-Associated MicroRNAs
Dysregulation of inflammation in degenerative disc disease, which is believed to be associated with pain development, is based on complex molecular mechanisms. One of the main regulators of inflammation are Toll-like receptors (TLRs), amongst which TLR2 is specifically relevant in disc pathophysiology. Importantly, inflammation is closely linked with mechanical loading in the IVD as high loads not only induce inflammation themselves, but also modulate TLR expression and lead to the accumulation of damage-associated molecular patterns with inflammatory/TLR activating capacity. As TLRs are an essential component to tissue inflammation, their tight control is crucial. In numerous cell types, microRNAs (= small noncoding RNAs) have been shown to modulate TLR signaling and the downstream inflammation and catabolism, both in health and disease. This project will be the first to identify and comprehensively investigate miRNAs in the context of TLR activation in the IVD and will thus elucidate posttranscriptional regulation of genes associated with IVD pathophysiology under the linked concepts of inflammation and mechanoregulation.
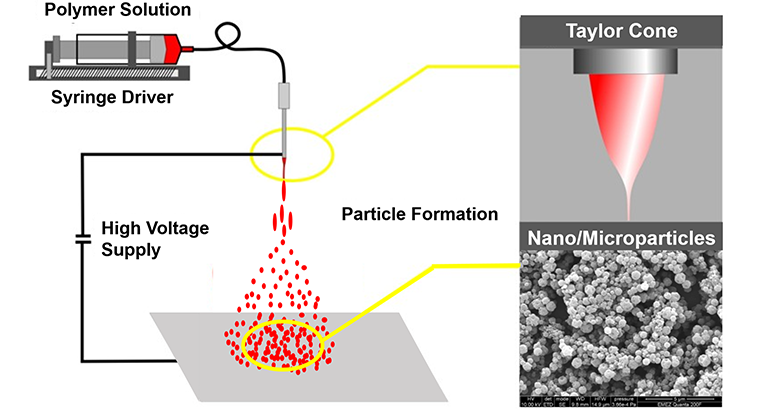
Electrosprayed Biomaterials for Drug Delivery
While oral delivery of pharmaceutical compounds generally has the highest compliance rates, local delivery of drugs can often be more effective. To prolong the effects of the selected therapeutics, such as substances with anti-inflammatory, anti-catabolic, and/or anti-oxidant properties, slow-release systems that provide sustained delivery and enhanced protection against degradation are needed. In this context, electrospraying is particularly useful as it is a gentle electrohydrodynamic encapsulation method that can be conducted under less harsh conditions than most other encapsulation techniques and is hence specifically suitable for sensitive substances such as natural polyphenols. In this project, we aim to create electrosprayed nano-/microparticles for improved drug delivery. We currently focus on encapsulating the polyphenol resveratrol for improved wound healing, but this approach is also relevant for other drugs and application areas.