Vinay Abhyankar
PhD Program Director, Biomedical and Chemical Engineering
Vinay Abhyankar
PhD Program Director, Biomedical and Chemical Engineering
Education
BS, Binghamton University; Ph.D., University of Wisconsin
Bio
Prof. Vinay Abhyankar received his B.S. in Mechanical Engineering from Binghamton University and his M.S. and Ph.D. in Biomedical Engineering from the University of Wisconsin–Madison, where he developed technologies to study cell motility in 3D environments. He then completed a postdoctoral fellowship at Sandia National Laboratories in Livermore, CA, where he designed molecular diagnostic techniques and modular microphysiological (organ-on-a-chip) systems.
Currently, he is an Associate Professor in the Department of Biomedical Engineering at RIT and serves as the Director of the Biomedical and Chemical Engineering PhD program. His research laboratory, funded by the NIH and NSF, operates at the interface of engineering, biomaterials, and biology. Current focus areas include: i) developing 3D tissue models to investigate how complex microenvironment cues direct cell positioning during disease progression, and ii) creating instrumented microphysiological barrier tissue models to study endothelial and tissue dysfunction.
These technology platforms are ideal for both undergraduate and graduate research involvement, combining simulation-based design, prototyping, and experimental studies to better understand human disease.
Select Scholarship
RIT students are in bold
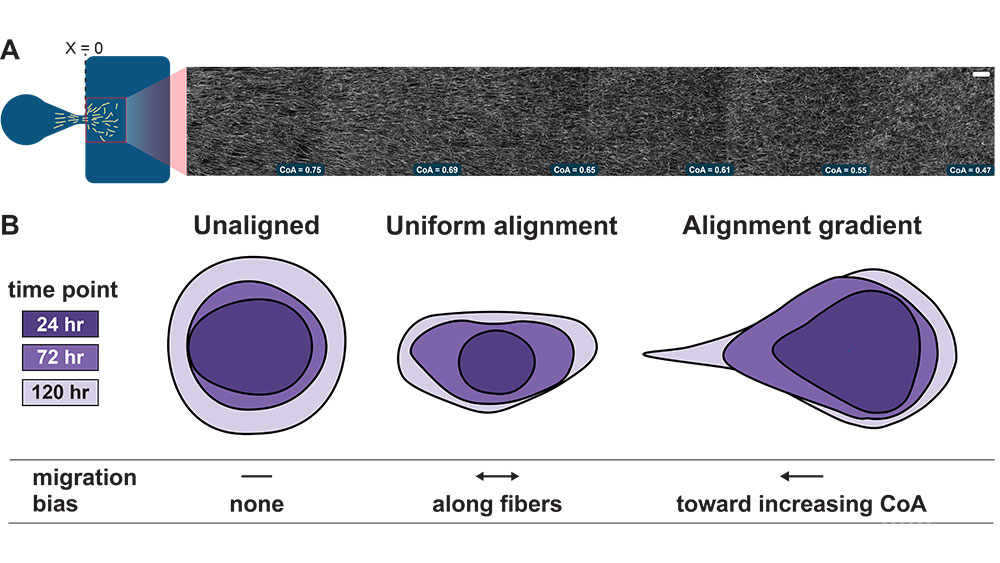
Joshi, I.M., Mansouri, M., Ahmed A., Simon, R.A., Esmaili, P., Desa, D.E., Elias T.M., Brown III E.B., Abhyankar, V.V. (2023).Microengineering 3D Collagen Matrices with Tumor-Mimetic Gradients in Fiber Alignment, Advanced Functional Materials, https://doi.org/10.1002/adfm.202308071
Mansouri M, Hughes A.R., Audi L.A., Carter A.E., Vidas J.A., McGrath J.L., Abhyankar V.V., 2023 Transforming Static Barrier Tissue Models into Dynamic Microphysiological Systems. JoVE https://www.jove.com/t/66090/transforming-static-barrier-tissue-models-into-dynamic
Lomeli-Martin, A, Ahamed, N., Abhyankar, V.V.,* Lapizco-Encinas, B.H.* (2023). Electropatterning - Contemporary developments for selective particle arrangements employing electrokinetics. Electrophoresis 44 884-909,https://doi.org/10.1002/elps.202200286, *corresponding authors
Ahmed, A., Joshi, I. M., Goulet, M. R., Vidas, J. A., Byerley, A. M., Mansouri, M., Day, S. W., & Abhyankar, V. V. (2022). Microengineering 3D Collagen Hydrogels with Long-Range Fiber Alignment. J. Vis. Exp., e64457. https://doi.org/10.3791/64457
Mansouri, M., Ahmed, A., Ahmad, S. D., McCloskey, M. C., Joshi, I. M., Gaborski, T. R., Waugh, R. E., McGrath, J. L., Day, S. W., & Abhyankar, V. V. (2022). The Modular μSiM Reconfigured: Integration of Microfluidic Capabilities to Study in vitro Barrier Tissue Models under Flow. Advanced Healthcare Materials, 2200802. https://doi.org/10.1002/adhm.202200802
McCloskey, M. C., Kasap, P., Ahmad, S. D., Su, S.-H., Chen, K., Mansouri, M., Ramesh, N., Nishihara, H., Belyaev, Y., Abhyankar, V. V, Begolo, S., Singer, B. H., Webb, K. F., Kurabayashi, K., Flax, J., Waugh, R. E., Engelhardt, B., & McGrath, J. L. (2022). The Modular µSiM: A Mass Produced, Rapidly Assembled, and Reconfigurable Platform for the Study of Barrier Tissue Models In Vitro. Advanced Healthcare Materials, 2200804. https://doi.org/10.1002/adhm.202200804
Hsu, M.-C., Mansouri, M., Ahamed, N. N. N., Larson, S. M., Joshi, I. M., Ahmed, A., Borkholder, D. A., & Abhyankar, V. V. (2022). A miniaturized 3D printed pressure regulator (µPR) for microfluidic cell culture applications. Scientific Reports, 12(1), 10769. https://doi.org/10.1038/s41598-022-15087-9
Ahmed, A., Mansouri, M., Joshi, I. M., Byerley, A. M., Day, S. W., Gaborski, T. R., & Abhyankar, V. V. (2022). Local extensional flows promote long-range fiber alignment in 3D collagen hydrogels. Biofabrication, 14(3), 035019. https://doi.org/10.1088/1758-5090/ac7824
Currently Teaching
In the News
-
December 20, 2023

RIT researchers develop new technique to study how cancer cells move
In tumors, cells follow microscopic fibers, comparable to following roads through a city. Researchers at Rochester Institute of Technology developed a new technique to study different features of these “fiber highways” to provide new insights into how cells move efficiently through the tumor environment.
-
March 22, 2023

RIT honors 14 researchers added to prestigious PI Millionaires group
RIT faculty members, who led research initiatives as principal investigators, were honored at a reception on March 21 to celebrate the individuals who helped the university reach record awards surpassing $92 million and place among the top private research universities in the country.
-
July 25, 2022

Vinay Abhyankar receives NSF grant to assess cancer cell migration processes
Cancer spreading from the primary tumor location to another is called metastasis and is the leading cause of cancer-related death worldwide. Research efforts today focus on discovering the guidance cues, or indicators, that promote movement of cancer cells toward blood vessels during early metastasis, and some of that work is taking place at RIT and the University of Rochester.
Featured Work
Studying fiber alignment gradients
Indranil M. Joshi
Despite their biological relevance, fiber alignment gradients had not been extensively studied in the lab. Our research addresses this gap by developing a new technique using microfluidic devices...