Collaborative Institutional Training Initiative (CITI) Training
CITI Training
Research at RIT is expected to be conducted to the highest ethical and professional standards. As one pursues research and trains the next generation, exposure to these concepts is crucial for excellence in research. To support this aim, RIT subscribes to the Collaborative Institutional Training Initiative (CITI Program) for training such as human subjects, ethical and responsible conduct of research, PHS/NIH Conflict of Interest, and others as necessary.
CITI Program is a web-based training employed by academic institutions, government agencies, and commercial organizations worldwide. Individuals receiving from and participating on awards from the National Science Foundation (NSF), National Institutes of Health (NIH), Public Health Sciences (PHS), and some other federally funded awards will need to show evidence of successful completion of applicable courses from the CITI Program. The training is generally valid for four years. Certifications obtained while at another institution may be honored with copies of current certificates. Please note that NSF, NIH and PHS awards currently cannot be released by Sponsored Research Services and Sponsored Programs Accounting until PIs and their teams complete applicable training. Novelution updates nightly with a listing of users who have are completed training and received certificates.
Follow these steps to register with CITI and enroll in the required course. CITI also offers a Quick Guide for New Learners with screen shots.
Register with CITI
- Go to the CITI homepage.
- Click the Register button in the top right corner of the page.
- In the “Select your Organization Affiliation” box, start typing “Rochester Institute of Technology” and select it when you see the name come up.
- Click the checkboxes to agree to the Terms of Service and affirm that you are an affiliate of RIT. Click the “Create a CITI Program account” button.
- Enter your personal information. Click “Continue to Step 3”
- Create a username, password and select a security question/answer. Click “Continue to Step 4”
- Ignore the question about ORCID ID. Provide country of residence, answer the contact question, then click “Finalize Registration.”
Choosing curriculum
Once logged in, users will have to answer several questions before courses are made available. Each question relates to a distinct curriculum: Human Subjects Research, Good Clinical Practices, Responsible Conduct of Research, PHS/NIH Conflict of Interest, and several others. Only answer the questions which apply to the training in which you are enrolling. If a module is not required, check the options for “Not At This Time/No” for the unrelated questions.
- As an example, if you are a physics professor and signing up for the Responsible Conduct of Research training, you would only answer Question 3 in the screen shot below. When selecting the RCR module, choose the field that best matches your expertise.
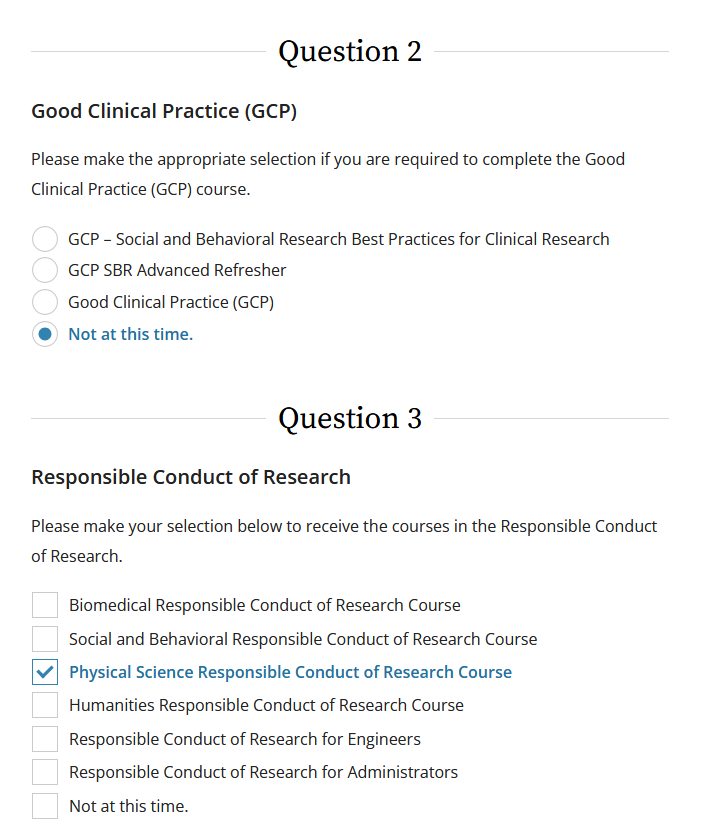
The questions assist in directing users to the appropriate training course(s). RIT has access to several modules that meet sponsor regulations and training requirements. We will occasionally add others as the need arises. You are free to explore the available curriculums. Once all questions are answered, click “Submit” at the bottom of the screen.
Course Completion
You can stop and start a course at any time; the next time you log in you will be at the point where you stopped.
Complete all components with a passing grade, and your completion will be recorded and stored by CITI. The Novelution system does a nightly sweep to see which RIT faculty and staff have completed CITI courses. Print a copy of the completion report for your records.
Keep your login and password information for future reference. The certificate will be valid for four years. Users may access their certificates at any time by going to My Reports.


