Chemistry Seminar - Alcohol and Amine Derivatives Guide Reactions
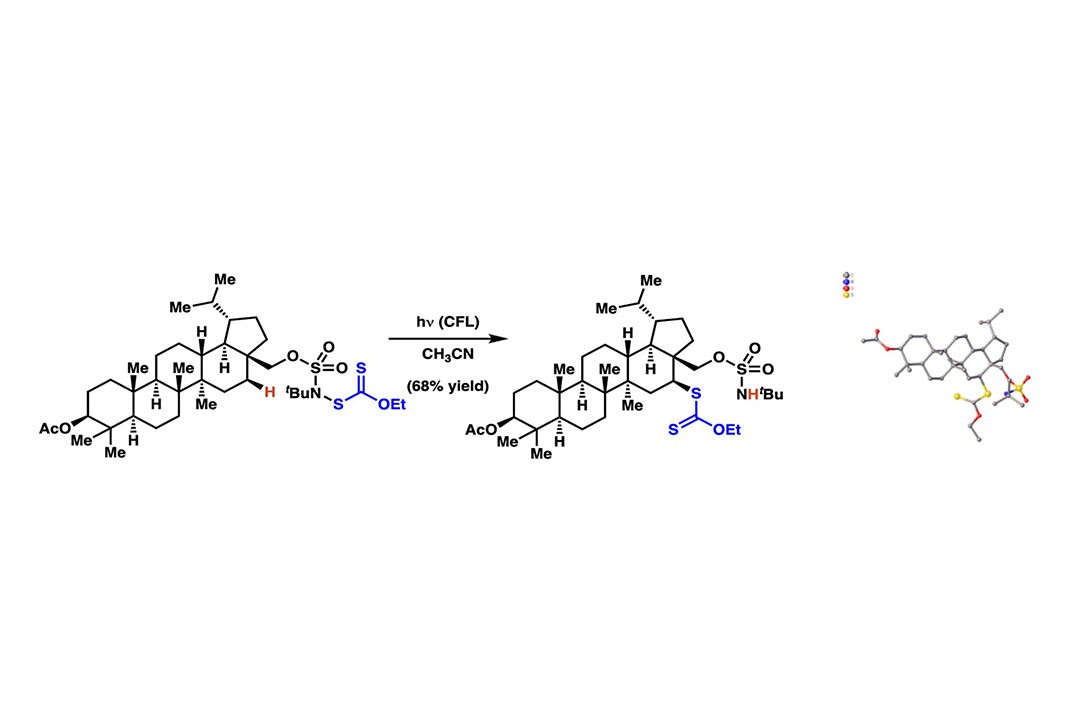
Alcohol and Amine Derivatives Guide Position-Selective C–H Functionalization ReactionsDr. Jennifer RoizenAssistant ProfessorDuke UniversityAbstract:In sophomore organic chemistry, everyone learns about epoxidation – a reaction inwhich an oxygen-atom is formally added across an alkene/olefin. This is an example of a broader class of reactions known as atom-transfer process, and in epoxidation, an oxygen-atom is the atom that is “transferred” to the olefin. An analogous process involves addition of an atom across a carbon–hydrogen bond such that the carbon–hydrogen bond is formally replaced with a new carbon–oxygen bond. This type of atom-transfer process has broad potential: it includes reactions to replace C–H bonds with carbon–oxygen, carbon–sulfur, carbon–chlorine or carbon–carbon bonds. In short, these atom-transfer reactions are powerful technologies to streamline access to health-relevant small molecules.Over the last decade, some of the most important advances in atom-transfer have originated from new platforms to control the site of a reaction – to selectively replace a targeted carbon–hydrogen bond in a molecule, without affecting 10–30 other pendant carbon–hydrogen bonds. Still, the utility of known C–H functionalization processes remains constrained by incomplete positional control in these reactions. This talk will focus on a novel hypothesis-driven strategy to control the position of atom-transfer using masked alcohols or amines to guide the process. This approach has the potential to substantively extend the synthetic utility atom-transfer reactions. The physiochemical basis for reactivity and positional-control will be discussed.Speaker Bio:Jennifer L. Roizen is an Assistant Professor at Duke University and a 2017 Thieme Chemistry Journals Award recipient. She had her first taste of synthetic research with J. Hodge Markgraf and Tom Smith as a Williams College undergraduate, where she advanced syntheses of benzoisocanthenones and contributed to publications on the total synthesis of hennoxazole A (a marine natural product). She moved to the California Institute of Technology to earn a Ph.D. with Brian Stoltz, researching approaches to access the ineleganolide core. These Cope-centric approaches remain the only published strategies to access the all carbon framework of ineleganolide, a small molecule that continues to elude synthetic campaigns. Upon graduation, Dr. Roizen became an NIH postdoctoral researcher and CMAD fellow with Justin Du Bois at Stanford University, where they extended intermolecular amination technologies. Dr. Roizen’s laboratory researches total synthesis and the development of cross-coupling and C– H functionalization processes.
Event Snapshot
When and Where
Who
Open to the Public