Chemistry Seminar -- Breaking Down That Inorganic-Organic Barrier
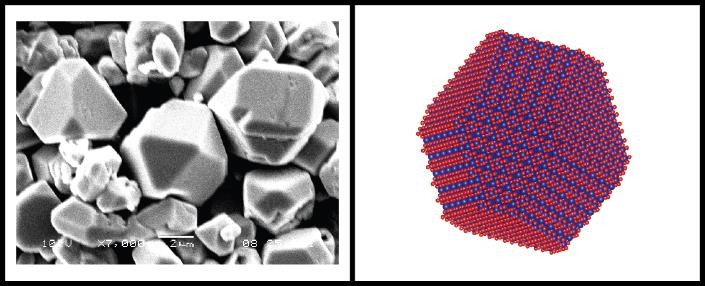
Functional Metal Oxide Shape Design Using a Non-hydrolytic Sol-Gel Approach: Breaking Down That Inorganic-Organic BarrierDr. Scott WilliamsProfessor of Inorganic ChemistrySchool of Chemistry and Materials Science, RITAbstract:Do the elements know the location of the inorganic-organic divide? Metal oxides are materials which function in almost every creature comfort we enjoy – our electronics, the batteries that power them, solar collection, and the sunscreen used at the beach. Fantastic changes in material or physical properties are realized when the nanomaterial is reduced in size from the micro to the nano-level. The challenge is to synthesize a metal oxide with a very narrow size and shape distribution. Quantum dot synthesis, the growth of quantum-sized CdS, elegantly demonstrates the Three Bears Principle of nanoparticle design – the particle may neither be too big nor too small, but just right to obtain optimized function. Metal oxides are traditionally grown using a sol-gel approach in water, because largely, that is the way Mother Nature has done it for Millennia; and, it just seems like the Inorganic thing to do. Synthetic metal oxide reaction mechanisms mediated by water, however, tend to be hydrolysis-condensation processes that happen way too fast and hard to control. What if we imagine growing the metal oxide in an organic solvent? In an organic solvent, the precursors, conditions, catalysts and mechanisms are changed. Instead of hydrolysis and condensation, the mechanism to a metal oxide nanoparticle is described using terminology such as ester, ether or alkyl halide eliminations, enol-keto tautomery and other processes that reside in the Organic domain. Non-hydrolytic sol-gel reaction schemes, using organic solvent systems, will be discussed as a way to control the size and define the shape of a select group of metal oxides. By controlling the shape, we can optimize the crystal facets that perform functional tasks such as ionic and electronic conductivity or chemical catalysis. Special focus will be on the shape design of cobalt oxide which is used in lithium ion batteries; and, barium titanate- the high dielectric metal oxide material used in advanced electronic technologies. As to whether there is an inorganic-organic divide? Perhaps, no longer.Speaker Bio:Professor Williams is currently a Professor of Inorganic Chemistry in the School of Chemistry and Materials Science at RIT. The Williams Group develops novel synthetic approaches to create printable inks that deposit conductive, semiconductive or resistive elements on a variety of form factors using production-scale print processes. Metal-oxide and metal organic decomposition systems (MOD) inks and structures are of particular interest in collaboration with the AMPrint Center.
Event Snapshot
When and Where
Who
Open to the Public