Biochemistry Bachelor of Science Degree

Biochemistry
Bachelor of Science Degree
- RIT /
- Rochester Institute of Technology /
- Academics /
- Biochemistry BS
Study the chemistry of life to prepare for careers in biotech, pharma, forensics, and agriculture.
$47.1K
Average First-Year Salary of RIT Graduates from this degree
10
Goldwater Scholars
25+
Lab spaces including teaching, research, and instrument labs
500MHz
Frequency of our in-house Nuclear Magnetic Resonance (NMR) spectroscopy instrument
Overview for Biochemistry BS
Why Study Biochemistry at RIT
Educational Growth: Follow in the footsteps of nearly half of RIT’s biochemistry bachelor’s degree students, who continued their education in graduate programs at top-tier universities, including Johns Hopkins University, Duke University, Yale University, Harvard University, MIT, Cornell University, and Virginia Tech.
Academic Communities: Join the student chapters of the American Chemical Society, Alpha Chi Sigma Professional Fraternity, or American Society for Biochemistry and Molecular Biology to connect with other students and professionals in your field, attend national conferences, and access employment and career resources.
Undergraduate Research: Engage in biochemistry research starting as early as your first year, preparing you for a wide range of careers with hands-on experience.
Direct Path to Medical School: RIT’s partnership with the University of Buffalo’s Jacobs School of Medicine offers eligible pre-health/pre-med students early admission and mentorship through the Early Opportunity Program in Medicine.
Jobs at Industry Leading Companies: Recent biochemistry graduates are employed at Ortho Clinical Diagnostics, St. Jude Children’s Research Hospital, ICON Laboratory, and Regeneron Pharmaceuticals, Inc.
Pre-Med/Pre-Health and Pre-Vet Advising Programs: Receive personalized guidance to become a competitive candidate for admission to medical and veterinary schools and graduate programs in the health professions.
STEM-OPT Visa Eligible: The STEM Optional Practical Training (OPT) program allows full-time, on-campus international students on an F-1 student visa to stay and work in the U.S. for up to three years after graduation.
Biochemists focus on the chemistry of life. RIT’s biochemistry bachelor’s degree provides knowledge in chemistry, biochemistry, and biology, which prepares you to consider real-world problems from a variety of perspectives. Delve deeper into your interest in combining the life and health sciences with RIT's chemistry degree.
RIT’s Biochemistry Bachelor’s Degree
The first year of the bachelor of science in biochemistry includes a mix of general biology and chemistry courses. During the upper-level years, you’ll take a substantial core of courses in biochemistry, physical chemistry, the liberal arts, and elective courses in life sciences. With RIT’s biochemistry BS you will be able to:
- Contribute your skills in corporate, health care, or government positions
- Enter professional education in medicine or other health-related fields
- Attend graduate programs in a variety of chemical and life sciences-related programs
Pre-Med/Pre-Health Advising
RIT’s Office of Pre-Health Advising offers an advising program that’s open to all majors and provides personal, individualized academic counseling to help you create a comprehensive long-term strategy to assist you in building successful applications to medical, dental, and veterinary schools or graduate degrees in the health professions (e.g., occupational therapy, physical therapy, etc.). Our pre-health advisors will have in-depth conversations with you around critical topics that include academic planning and course selection, MCAT and other admission exams, undergraduate research opportunities, clinical experiences and field work, timelines, and much more. Learn more about pre-med/pre-health advising.
Pre-Vet Advising
RIT’s pre-vet advising program provides personalized support to help you prepare successful applications for veterinary medical school. Pre-vet advising offers guidance on course selection, veterinary and animal care experience requirements, the veterinary school application process, and more. Learn about RIT’s pre-vet advising program.
Furthering Your Education in Biochemistry
Today’s careers require advanced degrees grounded in real-world experience. RIT’s Combined Accelerated Bachelor’s/Master’s Degrees enable you to earn both a bachelor’s and a master’s degree in as little as five years of study, all while gaining the valuable hands-on experience that comes from co-ops, internships, research, study abroad, and more.
- +1 MBA: Students who enroll in a qualifying undergraduate degree have the opportunity to add an MBA to their bachelor’s degree after their first year of study, depending on their program. Learn how the +1 MBA can accelerate your learning and position you for success.
Advanced Degrees
If you’re interested in pursuing medical school and/or graduate programs in the health professions, two advising programs can guide your planning for advanced study in these competitive areas of study:
- Chemistry and materials science and engineering graduate programs offered by the School of Chemistry and Materials Science prepare professional scientists by offering curricula that allow students to specialize in their chosen fields while engaging in rigorous, meaningful research using state-of-the-art instrumentation and facilities, under the guidance of a faculty mentor. The school offers the following advanced degrees: an advanced certificate in materials science and engineering, and master of science degrees in chemistry and materials science and engineering.
RIT/University of Buffalo’s Early Opportunity Program in Medicine
RIT pre-health/pre-med students have a direct path to medical school through an RIT partnership with the Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo (UB). The Early Opportunity Program in Medicine allows eligible RIT students to secure a pre-admission offer to the Jacobs School while completing their undergraduate degree at RIT. Students accepted into the program gain early access to professional training and mentorship at UB’s medical school, helping them prepare for the demands of medical school and beyond. Learn more about the Early Opportunity Program in Medicine.
-
Join Us for Accepted Student Open House
Visit campus on March 28 or April 11 to meet faculty, tour campus, and ask your questions.
-
Join us for Fall 2026
There's still time to apply. For some programs, applications will be reviewed on a rolling, space-available basis.
Careers and Cooperative Education
Typical Job Titles
| Computer-Aided Drug Design Chemist | Quality Control Analyst | Chemist |
| Research Technologist | Medical Student | Analytical Chemist |
| Forensic Scientist | Biotechnology Researcher | Health Scientist |
| Separations Chemist | Process Chemist | Cosmetic Biochemist |
| Food Biochemist |
Industries
-
Biotech and Life Sciences
-
Health Care
-
Higher Education
-
Medical Devices
-
Pharmaceuticals
Cooperative Education
What’s different about an RIT education? It’s the career experience you gain by completing cooperative education and internships with top companies in every single industry. You’ll earn more than a degree. You’ll gain real-world career experience that sets you apart. It’s exposure–early and often–to a variety of professional work environments, career paths, and industries.
Co-ops and internships take your knowledge and turn it into know-how. Science co-ops include a range of hands-on experiences, from co-ops and internships and work in labs to undergraduate research and clinical experience in health care settings. These opportunities provide the hands-on experience that enables you to apply your scientific, math, and health care knowledge in professional settings while you make valuable connections between classwork and real-world applications.
Cooperative education is optional but strongly encouraged for biochemistry majors.
Featured Work and Profiles
-
Connecting Biochemistry and Business: A Remarkable RIT Journey
Oreoluwa Fatimilehin found her path at RIT, combining science and business to build a successful career as a Product Attorney navigating tech’s legal challenges.
Read More about Connecting Biochemistry and Business: A Remarkable RIT Journey -
Biochemistry Student Receives Prestigious Goldwater Scholarship
Aidan Miller has been awarded the Barry M. Goldwater Scholarship, the most prestigious undergraduate research award in the United States. Miller is the tenth RIT biochemistry student to win the award....
Read More about Biochemistry Student Receives Prestigious Goldwater Scholarship -
VP of Research and Development in a Science-first Cannabis Company
Lauren Tamburro wanted to be acknowledged as a serious scholar, so she chose RIT. Today, she is the VP of Research and Development at Vertosa, Inc.
Read More about VP of Research and Development in a Science-first Cannabis Company -
Finding Work/Life Balance as a Podiatrist
Breeann Wilson ’02 always wanted to perform surgery, but she also wanted flexibility. Now she runs her own podiatry practice in her hometown and finds time to pursue personal interests.
Read More about Finding Work/Life Balance as a Podiatrist -
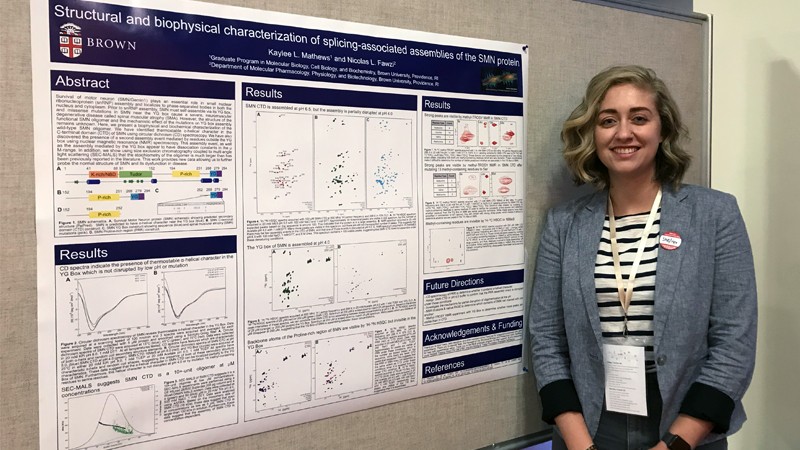
From Science Exploration to Brown University Researcher
Kaylee Mathews ’16 (science exploration/biochemistry) Kaylee Mathews ’16 discovered the exciting world of research during her year in RIT’s Science Exploration program. Kaylee recently earned her Ph.D. at Brown University.
Read More about From Science Exploration to Brown University Researcher -
No Matter Where You Start, RIT Gets You There
Niaya Jackson and Nana Aikins (biochemistry) Biochemistry majors Nana Aikins and Niaya Jackson found the supportive, close-knit community at RIT College of Science helped them figure out their next steps.
Read More about No Matter Where You Start, RIT Gets You There
Curriculum for 2025-2026 for Biochemistry BS
Current Students: See Curriculum Requirements
Admissions and Financial Aid
This program is STEM designated when studying on campus and full time.
First-Year Admission
First-year applicants are expected to demonstrate a strong academic background that includes:
- 4 years of English
- 3 years of social studies and/or history
- 3 years of mathematics is required and must include algebra, geometry, and algebra 2/trigonometry. Pre-calculus is recommended.
- 2-3 years of science is required and must include biology and chemistry.
Transfer Admission
Transfer applicants should meet these minimum degree-specific requirements:
- A minimum of college algebra is required. Pre-calculus or calculus is preferred.
- Chemistry and biology are required.
Financial Aid and Scholarships
100% of all incoming first-year and transfer students receive aid.
RIT’s personalized and comprehensive financial aid program includes scholarships, grants, loans, and campus employment programs. When all these are put to work, your actual cost may be much lower than the published estimated cost of attendance.
Learn more about financial aid and scholarships
Accreditation
Research
Undergraduate Research Opportunities
Many students join research labs and engage in research starting as early as their first year. Participation in biochemistry research leads to the development of real-world lab techniques, enhanced problem-solving skills, and broader career opportunities. Our students have opportunities to travel to national conferences for presentations and also become contributing authors on peer-reviewed manuscripts. Explore the variety of chemistry undergraduate research happening across the university.
Related News
-
August 1, 2025

RIT undergraduates share the impacts of their research
At the Undergraduate Research Symposium, RIT students share research in artificial intelligence, sustainability, health sciences, and other areas that is helping to solve global challenges.
-
May 27, 2025

Thirteen seniors at partner high school headed to RIT
RIT’s partnership with Rochester Prep High School celebrated its 10-year anniversary.
-
April 17, 2025

Two College of Science students earn Goldwater Scholarships
Grace Perna, a third-year biotechnology student, and Eva Reilly, a second-year biochemistry major, will use the scholarship to further their research in labs that aim to find better solutions for a range of diseases.
Contact
- Michael Heagy
- School Head
- Dean’s Office
- College of Science
- 585‑475‑2090
- mdhsch@rit.edu
School of Chemistry and Materials Science